Healthcare Provider Portal User Guide
On this page:
1. About the Healthcare Provider Portal
3. Who can use the Healthcare Provider Portal?
4. Accessing the Healthcare Provider Portal
5. Requesting and approving delegate access
6. Search for a patient and see their screening dates
7. Submitting information to the NCSR
7.1. Submitting Program forms to the NCSR
7.2 Alternative Access to Kits model
– Bulk ordering bowel screening test kits
– Issue/reissue kits directly to your patients
1. About the Healthcare Provider Portal
The National Cancer Screening Register (NCSR) enables a single electronic record for each person participating in the Australian Government’s bowel, cervical and lung cancer screening programs (the screening programs).
The intention of this guide is to assist users to communicate and interact with the NCSR through the Healthcare Provider Portal (the Portal).
The Portal allows those with a Provider Digital Access (PRODA) account to interact with the NCSR in order to:
- Search for a participating patient and view their test results and screening histories
- Submit information and diagnostic forms to the NCSR
- View and update your patient’s contact and demographic details
- Manage your patient’s participation in the screening programs, including opt out, resume participation, defer screening or cease your patient’s correspondence for the screening programs
- Nominate other people to assist your patient (such as a personal representative or another healthcare provider)
- Request replacement bowel screening test kits on behalf of your patients
- Bulk order bowel screening test kits to your practice to issue to your eligible patients who may be overdue for screening, via the Alternative Access Model.
- Enrol a patient in the National Lung Cancer Screening Program (NLCSP)
Please note, your access to what you can do on the NCSR will depend on what type of provider you will be registering and logging in as.
For further assistance, you can call the contact centre on 1800 627 701. The contact centre operates Monday to Friday, between 8am and 6pm in all Australian state and territory time zones.
You can also access our Quick Start Guide for the Healthcare Provider Portal, which provides a condensed version to the guide below.
2. Walkthrough video guide
In this video series you’ll learn how to:
- Register access to the Portal and log in via PRODA
- Manage patient participation in the screening programs (including enrolling a patient in the National Lung Cancer Screening Program)
- Request and approve delegate access
- Manage your profile, and delegate access to providers without a Medicare Provider number.
3. Who can use the Healthcare Provider Portal?
The Portal is designed for general practitioners, nurses, specialists and lab staff to support people participating in the screening programs.
Previously, providers had to call the NCSR, or the relevant pathology laboratory, to request patient screening information. The Portal provides another avenue to securely access and submit bowel, cervical and lung cancer screening data electronically in a self-service fashion.
4. Accessing the Healthcare Provider Portal
This section will guide you through:
- accessing the Portal for the first time,
- requesting delegate access from a healthcare provider with a Medicare Provider number, and
- approving delegate access as a healthcare provider.
4.1. Logging into the Healthcare Provider Portal
Logging in using PRODA
Before you register for the Healthcare Provider Portal for the first time, you will need a Provider Digital Access (PRODA) account.
PRODA is an online authentication system used to securely access certain online services including the Health Professional Online Services (HPOS), the National Disability Insurance Scheme, and the Portal
For assistance with registering for a PRODA account, please see the PRODA Registration Guide or visit our frequently asked questions about PRODA under the For Healthcare Providers section.
- Once you have a PRODA account, access the login page
- Enter your PRODA Username and Password and select Login

4.2. Accepting the Terms and Conditions
Read the terms and conditions, then select Accept.

4.3. Selecting the type of user you are
In order to use the portal, you will be a:
- General practitioner, radiologist or other specialist
- Pathologist
- Delegate of one of the above roles
- Cervical non-Medicare Provider Nurse with an identification number.
Select the type of user you are from the following options:
- You have a Medicare provider number
- You have a State and Territories Access number (STAN) / Register Identification Number (RIN)
- You are practice or lab staff acting as a delegate of a healthcare provider with a Medicare Provider number.
Select Continue and select the type of user you are.
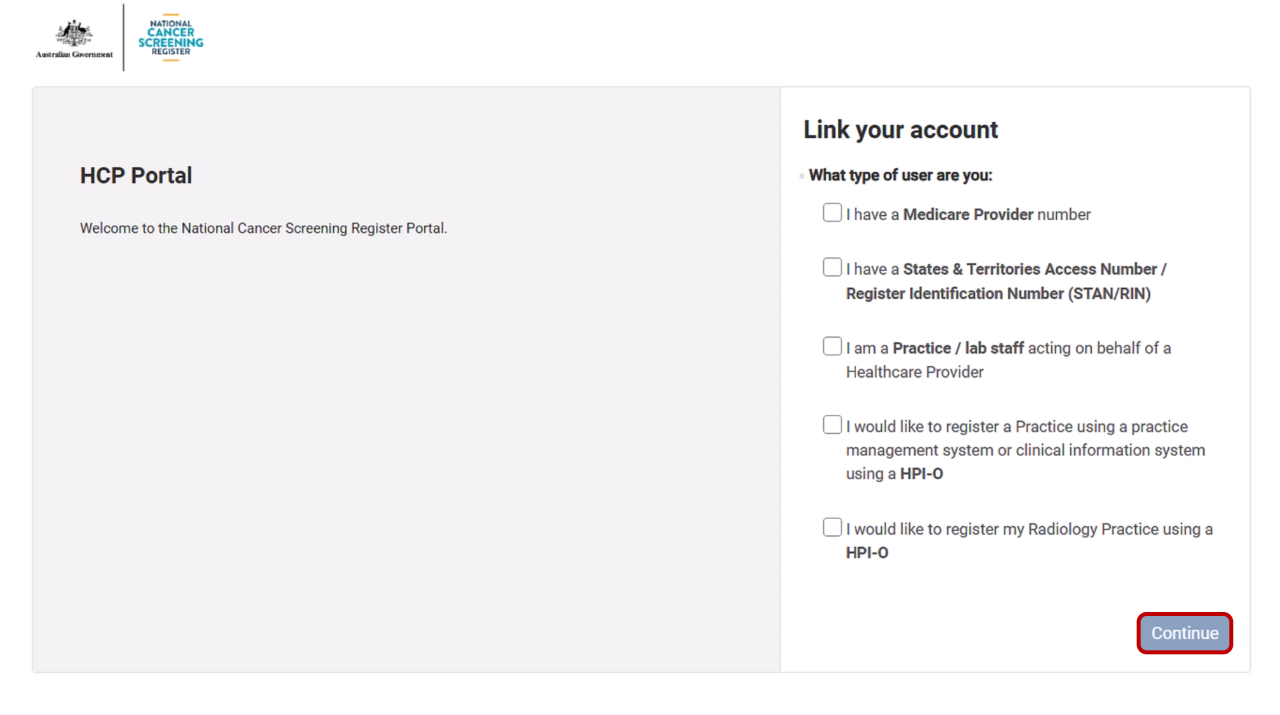
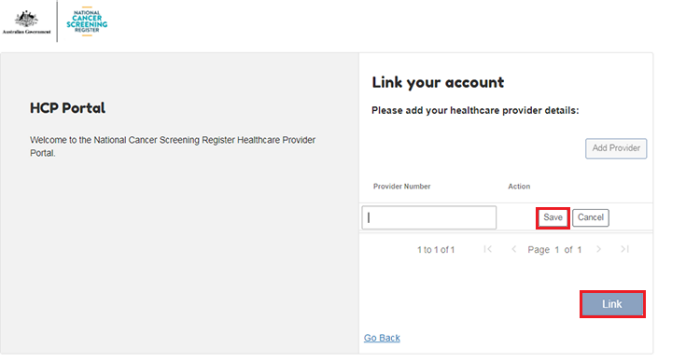
4.4. Link a provider number to the NCSR
Type in your medical identifier, select Save and then select Link. You will now be re-directed to the Portal’s Search Participant page.
In order to link multiple provider numbers to the NCSR, select Add provider.
If you are a practice staff/lab user, view how to get delegated access.
5. Requesting and approving delegate access
Delegated access allows those without a Medicare Provider number or STAN / RIN to gain access to the NCSR with the permission of either a healthcare provider, radiologist or a pathologist. An example of a person requiring delegated access could be a medical clinic’s administrative staff/practice nurse. This section will explain how to request this access, and how a Portal user can accept or reject these requests for access.
5.1. Requesting delegated access
Once you have chosen “I am a practice / lab staff acting on behalf of a healthcare provider” and clicked Select,
- Enter the provider number of the healthcare provider you would like to request access from and select Submit.
- The request will display as pending until the healthcare provider has accepted your request.
You will then be automatically logged out. You will need to contact the approving healthcare provider and ask them to login to the Portal to approve your request. Once your delegate access has been approved you will be able to successfully access the NCSR Portal.

5.2. Approving delegated access as a healthcare provider
Select My Profile.
Select Manage Delegation. From this page, you will be able to view who you have allowed access to the Portal and new requests for delegated access.
Requests for access will have a status of “Pending”. Select Approve to grant access to your delegate.

Once your account is linked, the next time you log in to PRODA you’ll see the NCSR tile listed in Available services:

6. Search for a patient and see their screening dates
This section outlines how a Portal user can search for their patient, view their eligible screening dates and their test results.
6.1. Searching for your participant
The Participant Search page requires you to enter the following information:
- Medical identifier – either a Medicare number, Individual Healthcare Identifier (IHI), Department of Veterans Affairs (DVA) number or Register Reference Number (RRN) - Mandatory
- Family Name – Mandatory
- Given Name – Optional
- Sex – Mandatory
- Date of Birth – Mandatory.
Populate the patient’s details in the fields as required and then select Search.
If the search details exactly match a participant on the NCSR, then their result will populate under Participant list. Only one result will be returned. Select the relevant screening program to navigate to the patient’s NCSR record.
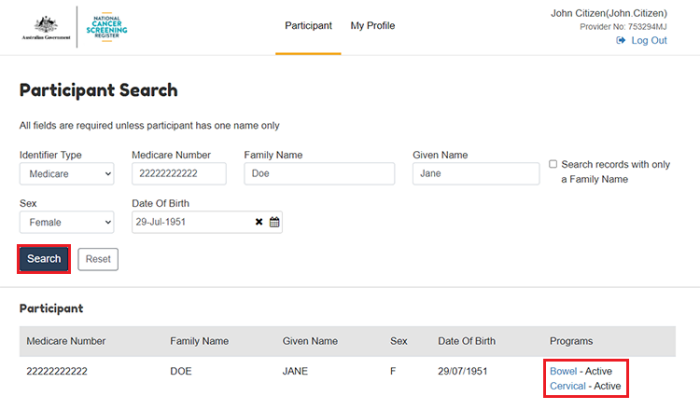
6.2. View your patient’s eligible screening action
When you have opened a patient record, you can view the patient’s screening information, including the Next Screening Action for any programs your patient is participating in.

The following Next Screening actions may be displayed for the participant:
Screening Action |
Description |
|---|---|
Bowel Program |
|
Deceased |
Participant is recorded in the NCSR as deceased. |
Inactive |
Participant has been discharged from the screening program due to colectomy, confirmed diagnosis, Medicare restriction, participant has opted out or is not eligible for the bowel program. |
Ineligible |
Participant is under the National Bowel Cancer Screening Program’s eligible age. |
Age Exited |
Participant is over the National Bowel Cancer Screening Program’s eligible age. |
Age Exiting |
Participant is currently eligible, but will be over the National Bowel Cancer Screening Program’s age eligibility at their next screening invitation round. |
Over Due (never screened, eligible since 50th birthday) |
Participant has been previously invited and never screened as part of National Bowel Cancer Screening Program. |
Due Now (eligible since 50th birthday) |
Participant is a newly eligible person who has turned 50 years of age, but has not yet completed the National Bowel Cancer Screening Program screening kit. |
Due Now (newly enrolled, eligible now) |
Participant is newly eligible with Medicare and is now registered for the National Bowel Cancer Screening Program. The participant has not yet completed a program kit. |
Skipped Round (eligible on: [Date]) |
Participant is currently skipping a round (i.e. due to recent colonoscopy). Displays the date when the participant will be next eligible to screen for the National Bowel Cancer Screening Program. |
Eligible on: [Date] |
Participant has previously screened with the National Bowel Cancer Screening Program. This is the date they are next eligible to screen. |
Eligible since: [Date] |
This participant has not recently screened with the National Bowel Cancer Screening. This is the date the participant has been eligible/due since. |
Cervical Program |
|
Deceased |
Participant is recorded in the NCSR as deceased. |
Hysterectomy |
Participant has been discharged from the screening program due to hysterectomy. |
Cancer – Exited |
Participant has the “Cervical Cancer” alert set, and is likely in clinical management. |
Ineligible |
Participant is under the National Cervical Screening Program’s eligible age. |
Age Exited |
Participant is over the National Cervical Screening Program’s eligible age. |
Overdue |
Participant has not screened as part of the National Cervical Screening Program before or has not screened in previous years and is eligible for screening. |
Due Now |
Participant is now eligible to start a new screening round as part of the National Cervical Screening Program. |
Commence at 25 |
Participant is under 24 years and 9 months, and has the status of "early screener", then their next action should state that they are due on their 25th birthday. |
Referral Recommended |
Participant referral to a specialist for further investigation is recommended. |
[Date] |
The date when the participant will next be due for to screen for the National Cervical Screening Program. |
Clinician-collected cervical sample for LBC needed. HPV (not 16/18) detected in self-collected vaginal sample |
Participant should return for a clinician-collected LBC after a self-collected Cervical Screening Test detected HPV (not 16/18).
|
Lung Program |
|
Deceased |
Participant is recorded in the NCSR as deceased. |
Inactive |
Participant is below eligible age, has a Medicare restriction or exclusion flag, has opted out or withdrawn consent to participate. |
Low-dose CT Unsuitable |
Participant not suitable for a low-dose CT scan. This may be temporary. |
Exited - diagnosed with cancer |
Participant is discharged from the screening program due to cancer detected. |
Age Exited |
Participant no longer meets the National Lung Cancer Screening Program age eligibility requirements (70 years). |
Age Exiting |
Participant is currently eligible but will no longer meet the National Lung Cancer Screening Program age eligibility requirements at their next screening reminder. |
Screening deferred until [date] |
Participant has deferred their next screening to a future date. |
Due Now |
Participant is newly enrolled by a healthcare provider in the National Lung Cancer Screening Program. The participant has not yet screened. |
Overdue: eligible since [date] |
Participant has been previously enrolled and never screened as part of National Lung Cancer Screening Program. |
Due since: [date] |
This participant has not recently screened with the National Lung Cancer Screening. This is the date the participant has been due since. |
Rescreen on: [Date] |
Participant has previously screened with the National Lung Cancer Screening Program. This is the date they are next eligible to screen. |
Referral recommended |
Participant referral to a specialist for further investigation is recommended. |
Referred to specialist - awaiting diagnosis |
Participant is referred to a specialist and awaiting further investigation. |
6.3. View patient alerts
From the participant section the alerts relevant to your patient will be displayed. These alerts are to indicate certain program result alerts, or administrative alerts such as screening deferral or mail delivery failures. Note that some alerts may persist over several screening rounds.

The following alerts may be displayed where applicable:
Bowel Program

Cervical Program

Lung Program
6.4. View your patient’s test results and correspondence
From the participant section you will be able to view the patient’s previous results by selecting Forms and then View on the row/ result you wish to open. The provider can also print these forms if required. In this view, you can also see the Outcome of the form or participant’s test result. By selecting Delete you can also remove a previously submitted form if you were the user who originally submitted it.
You can also select Filter to filter by Submitted Date or Document Name. Partial text can be entered in these search fields (for example “result”). Select Apply to search according to filter parameters.

From the participant section you will also be able to view the patient’s previous correspondence by selecting Correspondence and then View on the row you wish to open. Correspondence can also be printed as required.
You can also select Filter to filter by Received Date or Document Name. Select Apply to search according to filter parameters.
7. Submitting information to the NCSR
This section explains how to locate a program form for submission to the NCSR for a patient.
You can use these forms to:
- Submit program Diagnostic Forms to the NCSR
- Nominate a healthcare provider on your patient’s behalf
- Nominate a Personal Representative on your patient’s behalf
- Manage your patient’s participation including requesting replacement kits for the bowel program
- Edit your patient’s details
To navigate to the forms from the patient profile, select the program, either Bowel, Cervical, or Lung, you would like to submit a form for, and then Forms.
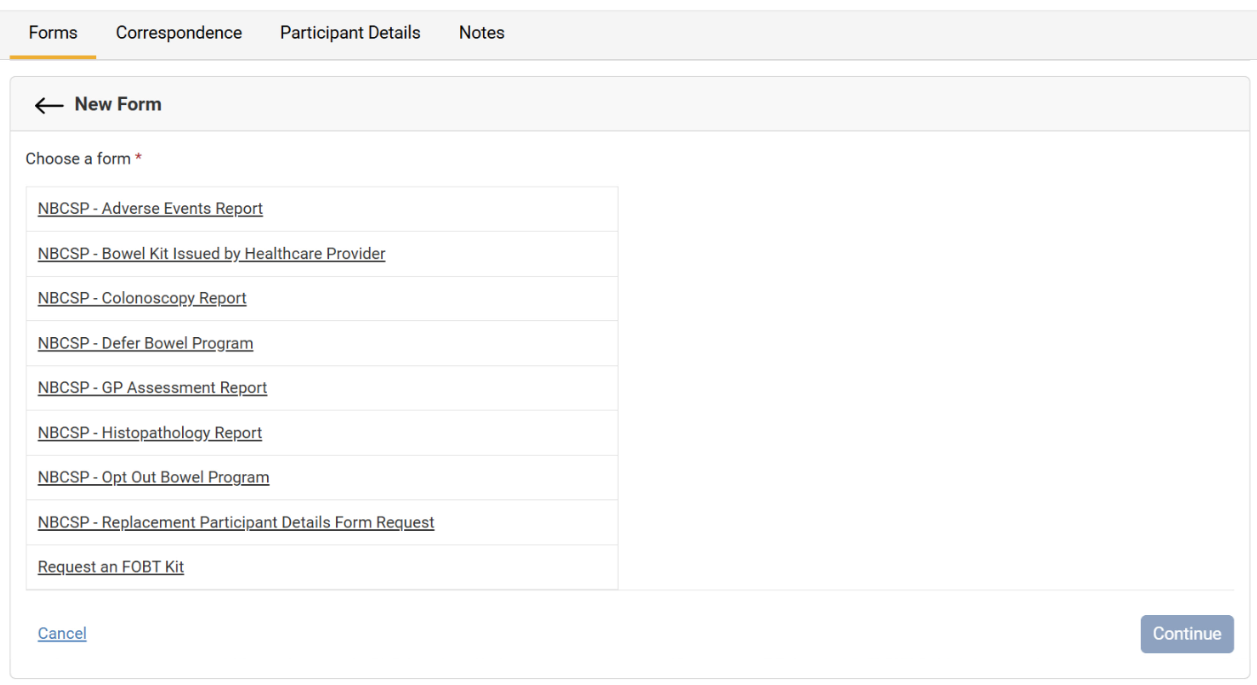
Select Choose a form.
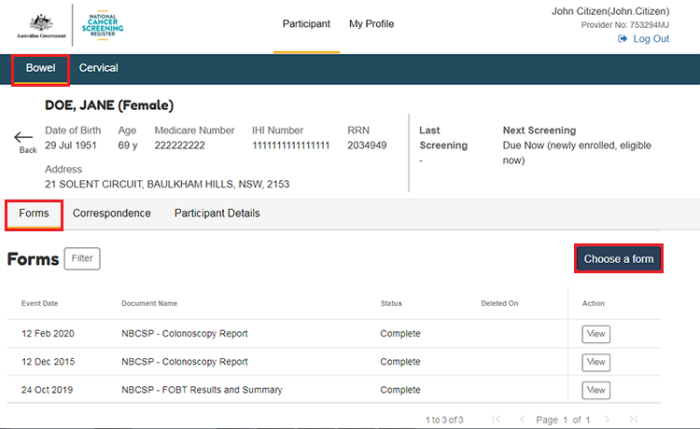
From the New Form page, select the form to be filled out by selecting the Form Name.
Select Continue, and when the form is complete, select Submit.
7.1. Submitting Program forms to the NCSR
This section explains what forms are available to submit clinical information regarding a patient to the NCSR.
In the Portal, the following forms will be available, based on your role, to submit:
Bowel Program |
|
NBCSP - Adverse Events Report |
To be completed by colonoscopists or other healthcare providers to report information about an adverse outcome for a procedure relating to diagnostic investigation of a program participant who has received a positive iFOBT result. This form is only used where that information has not already been included in the Colonoscopy Report. |
NBCSP - GP Assessment Report |
To be completed by general practitioners to provide information about consultations with program participants who have received a positive iFOBT result. This supports timely follow-up by the NBCSP along the screening pathway. |
NBCSP - Colonoscopy Report |
To be completed by colonoscopists to report the results of a colonoscopy for a Program participant with a positive iFOBT result. This form also enables colonoscopists to report adverse events which may have occurred during the procedure or are known at the time of completing this form. |
NBCSP - Histopathology Report |
To be completed by a histopathologist or a colonoscopist to report the results from testing specimens collected during the colonoscopy procedure for a program participant with a positive iFOBT result. |
Cervical Program |
|
NCSP - Abnormal Result Questionnaire |
This report is to provide information to the NCSR where a program participant has had abnormal cervical screening results, and the NCSR has not received notice that a colposcopy has been performed. The National Cervical Screening Program will send a letter to the healthcare provider to request this information where applicable. |
NCSP – Colposcopy and Treatment Form |
To be completed by a colposcopist to notify prescribed cervical screening information to inform participant clinical pathways and support the safety net function of the NCSR. It is also used for reporting performance measures for colposcopists. |
NCSP – Total Hysterectomy Assessment Form |
To be completed by healthcare providers to notify the NCSR when a patient has had a total hysterectomy. This will ensure the hysterectomy is accurately reported against their record, and no further invitations and reminders for cervical screening will be sent to them from the NCSR. |
Lung Program |
|
NLCSP – Eligibility & Enrolment Form |
A requesting practitioner completes a low-dose CT scan request for an eligible patient and submits the Eligibility & Enrolment form to initiate the screening journey for their patient. |
NLCSP - Participation Management Form |
Update your patient’s screening status – such as confirming if they should exit the NLCSP due to becoming ineligible, opting out or being diagnosed with lung cancer. If there are changes to their suitability to low-dose CT for their follow-up scan, this can also be updated through this form. Keeping this up to date helps avoid unnecessary follow-ups if they needed to be exited or ensures the register safety net is resumed if they again become suitable for low-dose CT scan. |
NLCSP - Biopsy Adverse Events Form |
Report any adverse events following a biopsy. This supports patient safety and informs clinical decision-making and contributes to quality and monitoring of the NLCSP. |
NLCSP - Specialist Referral Form |
Notifies the register of referral of patients with a cat 5/6 high-risk low-dose CT scan result to a respiratory physician or other specialist. Prompt notification will avoid the NCSR contacting you to seek information to ensure your patient is getting timely investigation of results and potential diagnosis. |
NLCSP - Diagnosis Form |
Record confirmed diagnostic findings after a low-dose CT scan. Accurate reporting supports future screening decisions such as whether to exclude or continue screening, timely care, and national reporting and quality improvement. |
NLCSP - Radiology Dataset |
Used by radiology practices to submit low-dose CT scan results, including provider details, nodule findings, and screening category, to support clinical follow-up, program monitoring, and appropriate patient management. |
View how to navigate to the Submit Forms page in the Portal.
7.2. Alternative Access to Kits Model
The Alternative Access to Kits Model allows health professionals to issue bowel screening kits to their patients directly. By recommending and explaining the test, healthcare providers can help to remove barriers to screening for some people.
The Healthcare Provider Portal enables you to:
- bulk order bowel screening test kits (iFOBT) directly to your practice
- record when you have issued a kit to an eligible patient, and
- track whether your patient has not yet returned their samples for testing so you can encourage them to complete the test.
Bulk ordering bowel screening test kits
At the top of the Healthcare Provider Portal page, select iFOBT Bulk Order.

Before ordering kits for the first time, you must complete some short training to understand the Alternative Access to Kits model and your role in it.
Once you’ve done the training, click on Start order.
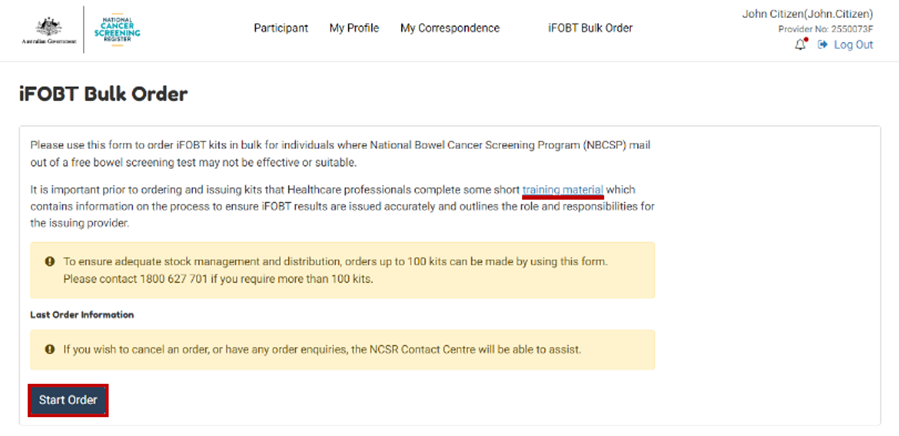
The Portal will pre-populate several fields, however you will need to enter your clinic name and phone number.
While the address of the clinic will pre-populate, a different delivery address can be entered (which won't change the address held in Medicare, it will just be for this order).
Next, select the number of kits you want to order:
- They’re ordered in batches of 10, with the recommended number, per provider, being no more than 30 kits.
- More can be ordered if you have a large eligible patient base.
- You will also be asked how many healthcare providers the request is for, how many kits you have in stock, and if you do have kits in stock, whether any are expiring within four weeks.
- If you order over 40 kits per provider, you will be asked for a reason.
Click Next.

Then confirm your order by checking the acknowledgement and clicking Submit.

The next page will display your order confirmation. A tracking number will be displayed on the iFOBT Bulk Order page within 10 business days.
Issue kits directly to your patients
When you issue a kit to a patient during a consultation, it’s important to review their contact details held in the NCSR and update them as needed. This information will pre-populate your patient’s Participant Details form.
To record that you have issued a kit, go to the forms tab in your patient’s Bowel record, select the NBCSP – Bowel Kit Issued by Healthcare Provider form.
This will inform the NCSR that a kit has been issued and means the sample can be identified when being processed by the contracted pathologist. The form will not appear in the list if the patient is not due to complete a test kit or is ineligible.

Complete the form and ensure a nominated healthcare provider is listed to receive your patient’s results.

After submitting the NBCSP – Kit Issued by Healthcare Provider form, a new tab will display the pre-populated Participant Details Form. Print this form for the patient to sign and return with their samples.
If you need to re-print the Participant Details Form, navigate to your patient's profile, select the Bowel program > Forms > then select Print/Re-print Participant Details Form.
The reprint option will only be displayed for participants who have been given a kit via the Alternative Access Model.
If the participant’s details are different to what is in their NCSR record, you will be able to update them once you have selected the Re-print button before printing the form.
These details will pre-populate the newly generated Participant Details Form.
8. Managing your patient's information and participation
This section explains what forms are available to manage your patient’s participation in the screening programs via the NCSR. You must have your patient’s consent to submit these forms.
In the Portal, the following forms will be available, based on your role, to submit on behalf of your patient:
Bowel Program |
|
Form Name |
Form description |
NBCSP - Opt Out Bowel Program |
Use this form to cease your patient’s participation in the bowel program. Future bowel program results for the participant (screening test, dates and related procedure test results or diagnosis) are not accepted or recorded in the NCSR from the effective date, and no contact or correspondence is made or sent, including invitations to screen, reminder letters and follow-up actions. |
NBCSP – Resume Participation |
On behalf of the participant, request that they are to resume participation in the bowel program. |
NBCSP - Defer Bowel Program |
Electing your patient to not participate in a screening program for a defined period of time. They may return to the screening program any time after the deferment period has ended or at any earlier time if they withdraw a request or if a screening test result is received by the NCSR. |
Cervical Program |
|
NCSP - Opt Out Cervical Program |
Future cervical program results for the participant (screening test, dates and related procedure test results or diagnosis) are not accepted or recorded in the NCSR from the effective date, and no contact or correspondence is made or sent, including invitations to screen, reminder letters and follow-up actions. |
NCSP – Resume Participation |
On behalf of the participant, request that they are to resume participation in the cervical program. |
NCSP - Defer Cervical Program |
Electing your patient to not participate in a screening program for a defined period of time. They may return to the screening program any time after the deferment period has ended or at any earlier time if they withdraw a request or if a screening test result is received by the NCSR. |
NCSP - Cease Contact and Correspondence |
Request that your patient no longer receive contact or correspondence from the NCSR for the cervical program only. |
Lung Program |
|
NLCSP - Defer Lung Program Form |
To change your patient’s next screening date – for example, due to travel. |
NLCSP - Opt Out Lung Program Form |
End your patient’s participation in the NLCSP in line with their request. No reminders will be sent, and results won’t be recorded. Patients can resume participation through the Participant Portal, calling the NCSR, or at the request of their healthcare provider. |
NLCSP – Resume Participation Form |
On behalf of the participant, request that they are to resume participation in the lung program. |
NLCSP - Cease Contact and Correspondence |
Request that your patient no longer receive contact or correspondence from the NCSR for the lung program only. |
View how to navigate to the Submit Forms page in the Portal.
8.1. Updating your patient’s details
In the NCSR you will be able to change your patient’s details on their behalf. You will be able to do this by selecting the Participant Details tab. The details can be updated across multiple programs or for a selected program only. In this section you can also update your patient’s Indigenous status and country of origin details.
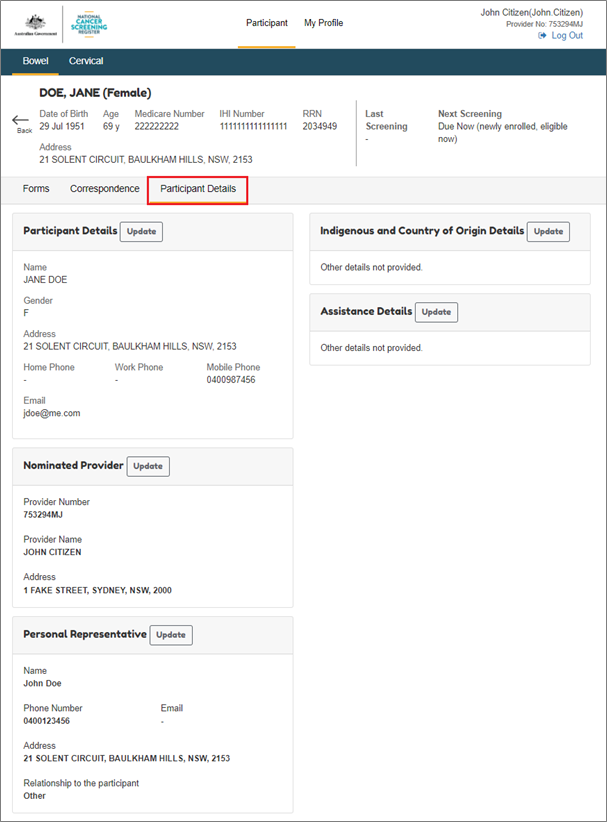
8.2. Nominate a healthcare provider on your patient’s behalf
In the NCSR, you will be able to nominate a healthcare provider for your patient to assist them and their participation in the program/s. You will be able to do this by selecting the Participant Details tab and going to the Nominated Provider section. The details can be updated across multiple programs or for a selected program only.
View how to navigate to the Participant Details tab in the Healthcare Provider Portal.
8.3. Nominate a Personal Representative on your patient’s behalf (bowel program only)
In the NCSR you will be able to nominate a personal representative for your patient to assist representing them and their participation. You will be able to do this by selecting the Participant Details tab and going to the Personal Representative section. The details can be updated across multiple programs or for a selected program only.
If your patient is participating in the cervical program, nominating a Personal Representative can be completed via the Request to Nominate a Personal Representative webform or by phoning the NCSR contact centre on 1800 627 702.
View how to navigate to the Participant Details tab in the Healthcare Provider Portal.
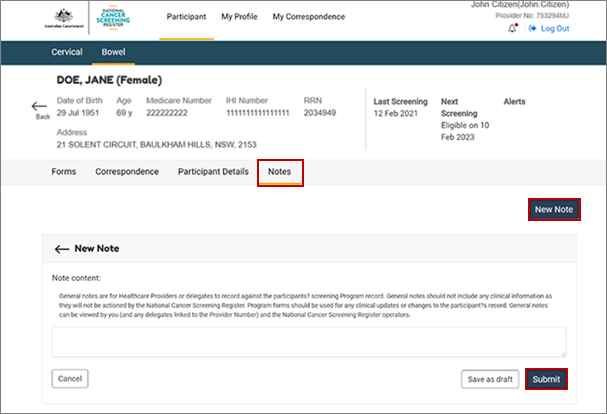
8.4. Submit general notes on your patient record
In the NCSR you will be able to save general notes to the patient record, by selecting the Notes tab. General notes are for healthcare providers or delegates to record against the participant’s screening program record. General notes should not include any clinical information as they will not be actioned by the NCSR. General notes can be viewed by you (and any delegates linked to the provider number) and the NCSR operators. Program forms should be used for any clinical updates or changes to the participant’s record.
You can save a draft progress note and save a final progress note.
8.5. View my correspondence
You can view correspondence sent to you from the NCSR, via the My Correspondence tab.
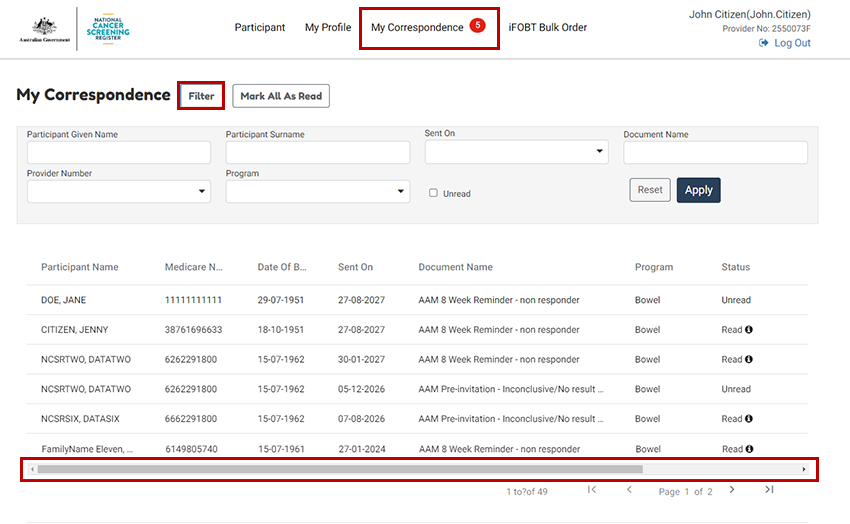
In this view you can view all correspondence that has been sent to you, across all patients.
9. Glossary of definitions
Term |
Definition |
30 Pack-Year Smoking History |
A measurement of tobacco exposure used to assess eligibility. A ‘30 pack-year smoking history’ equals smoking one pack of cigarettes per day for thirty years. Pack years are not an exact science and healthcare providers are encouraged to use clinical judgement. |
Bowel Participant |
A person who has a Bowel Program iFOBT result recorded on the NCSR and are considered an active participant in the program. |
Bowel Program |
The National Bowel Cancer Screening Program (NBCSP or bowel program). |
Bowel Program Eligibility |
Australians aged 45 to 74 are eligible for the immunochemical Faecal Occult Blood Test (iFOBT) free of charge through the NBCSP. |
Bowel Screening |
Bowel screening involves testing for bowel cancer in people who do not have any obvious symptoms of the disease. The aim is to find cancers early when they are easier to treat and cure. Bowel screening can also find polyps, which may develop into cancer over time. |
CAD/AI/Volumetry Software |
Computer-aided detection (CAD), artificial intelligence (AI), or volumetry tools used by radiologists to support interpretation of low-dose CT scans. |
Cease correspondence |
This is a request to the NCSR by an eligible person, their personal representative or healthcare provider, for no contact or correspondence from the NCSR for the cervical or lung programs. Ceasing correspondence is not an option for the bowel program. |
Cervical Participant |
A person who has any cervical cytology or histopathology test recorded on the NSCR. |
Cervical Program |
The National Cervical Screening Program (NCSP or cervical program). |
Cervical Program Eligibility |
Eligible women aged 25 years or over who have not yet started cervical screening will receive an invitation from the NCSR to have the new cervical screening test. |
Cervical Screening Test |
A Cervical Screening Test looks for signs of the human papillomavirus (HPV). Participants can either collect their own vaginal sample (self-collection), or have a healthcare provider collect a cervical sample (clinician-collected). |
Correspondence Preference |
The channels – such as text (SMS), email or letters – a participant of the NCSR would prefer to receive correspondence from for any programs they are participating in. |
Deferring participation |
This is a request to the NCSR by a person, their personal representative or healthcare provider, who has elected to not participate in a screening program for a defined period of time. They may return to the screening program any time after the deferment period has ended or at any earlier time if they withdraw a request or if a screening test result is received by the NCSR. |
Healthcare Provider Identifier – Individual (HPI-I) |
Healthcare Provider Identifier – Individual (HPI-I) is a medical identifier for healthcare professionals involved in providing patient care. |
Healthcare Provider Identifier – Organisation (HPI-O) |
Healthcare Provider Identifier – Organisation (HPI-O) – is a medical identifier for organisations that deliver healthcare (such as hospitals or general practices) |
Healthcare provider |
Has the same meaning as in the Healthcare Identifiers Act 2010 (Cth). A person associated with either a health specialty or a health discipline and who is qualified and certified by regulatory bodies to provide a healthcare service to a patient. For example, a general practitioner, other specialist, colonoscopist, colposcopist, histopathologist, pathologist, radiologist, nurse, midwife and Aboriginal Healthcare Worker. Note: healthcare provider is the preferred term for use in NCSR documentation as opposed to healthcare professional. |
Individual Healthcare Identifier (IHI) |
Is a medical identifier for individuals receiving healthcare services. |
Low-Dose Computed Tomography (Low-dose CT) |
A low-dose computed tomography (CT) scan uses a small amount of radiation to take detailed images of your lungs. It can detect lung cancer before symptoms appear and is used in the National Lung Cancer Screening Program for early detection. |
Lung Cancer Screening |
Lung cancer screening is a medical imaging procedure that checks for signs of lung cancer before symptoms appear. It is performed in a radiology setting using a low-dose CT scan for people at higher risk. Early detection can increase the chance of successful treatment. |
Lung Cancer Screening Participant |
A person who has a record of participation in the National Lung Cancer Screening Program, including a completed Eligibility and Enrolment Form, low-dose CT scan results, or other lung program-related clinical information recorded in the NCSR. |
Lung Program |
The National Lung Cancer Screening Program (NLCSP) aims to detect lung cancer early in people at high risk, using low-dose computed tomography (low-dose CT) scans to reduce illness and death from lung cancer. |
Lung Program Eligibility |
Eligible participants are aged 50 to 70 years, have no signs or symptoms of lung cancer, currently smoke tobacco cigarettes or have quit within the past 10 years, and have at least a 30 pack-year smoking history. |
Lung Program Screening Category |
A classification assigned after low-dose CT scan based on findings (using the NLCSP Nodule Management Protocol), indicating the participant’s risk of lung cancer and guiding next steps (e.g. continue routine screening, follow-up scan, or referral to specialist). |
NBCSP - Adverse Events Report |
To be completed by colonoscopists to report information about an adverse outcome for a procedure relating to diagnostic investigation of a Program participant who has received a positive iFOBT result. This form is only used where that information has not already been included in the Colonoscopy Report. |
NBCSP - Colonoscopy Report |
To be completed by colonoscopists to report the results of a colonoscopy for a program participant with a positive iFOBT result. This form also enables colonoscopists to report adverse events which may have occurred during the procedure or are known at the time of completing this form. |
NBCSP - GP Assessment Report |
To be completed by general practitioners to provide information about consultations with Program participants who have received a positive iFOBT result. |
NBCSP - Histopathology Report |
To be completed by a colonoscopist or histopathologist to report the results from testing specimens collected during the colonoscopy procedure for a Program participant with a positive iFOBT result. |
NCSP - Abnormal Results Questionnaire |
This report is to provide information to the NCSR where a Program participant has had abnormal cervical screening results, and the NCSR has not received notice that a colposcopy has been performed. The National Cervical Screening Program will send a letter to the healthcare provider to request this information where applicable. |
NCSP - HPV LBC PAP Results |
Only for reporting cervical screening tests where the laboratory does not transmit cervical screening test results to the NCSR electronically (by HL7 format). |
NCSP - Histology |
To be completed by a histopathologist to report on the results of cervical histology from a pathology laboratory. |
NCSP – Colposcopy and Treatment Form |
To be completed by a colposcopist to notify prescribed cervical screening information to inform participant clinical pathways and support the safety net function of the NCSR. It is also used for reporting performance measures for colposcopists. |
NLCSP - Biopsy Adverse Events Form |
Used to report adverse events following a biopsy performed as part of follow-up after lung cancer screening. This supports safety monitoring and quality improvement. |
NLCSP - Diagnosis Form |
Completed by a healthcare provider to report whether lung cancer is present or absent following diagnostic work-up after a high-risk low-dose CT scan. Information provided informs clinical follow-up and supports program safety net functions. |
NLCSP - Eligibility and Enrolment Form |
Used by a GP or medical practitioner to assess a patient’s eligibility and suitability for lung cancer screening and, if eligible to formally enrol them in the program. This form can also be used to:
|
NLCSP - Participation Management Form |
Used to update a participant’s status in the Lung Program. This includes confirming continued eligibility, withdrawing participation, or recording clinical reasons to cease screening. |
NLCSP - Radiology Dataset |
Used by radiology practices to submit structured clinical and scan data following a low-dose CT scan. It includes patient demographic information, referring provider information, scan quality, findings (including nodules) for lung and other actionable findings, and screening outcomes. |
NLCSP - Specialist Referral Form |
Used to refer patients with a high-risk low-dose CT result to a specialist (e.g. respiratory physician, multi-disciplinary team) for further assessment and timely clinical management. |
Nominated healthcare provider |
This is a request to the NCSR by an eligible person, their personal representative or healthcare provider, who wants to nominate a healthcare provider in relation to correspondence with the NCSR. The nominated healthcare provider is able to access details about the participant and will, in addition to the participant, receive reminders and follow up for screening. Nominating a healthcare provider can be set per program. |
Opt out |
A request to opt out of all participation in the NCSR means that forthcoming results (screening test, dates and related procedure test results or diagnosis) are not accepted or recorded in the NCSR from the effective date, and no contact or correspondence is made or sent, including invitations to screen, reminder letters and follow-up actions. |
PRODA |
PRODA (Provider Digital Access) is an online authentication system managed by Services Australia and used to securely access certain online services including Health Professional Online Services (HPOS) and the National Disability Insurance Scheme (NDIS). Designed as a two-step verification process, it requires a username, password and verification code to login. |
Participant |
This is an eligible person:
|
Personal Representative |
A personal representative may be appointed by a participant or may be authorised to act on behalf of a participant in relation to the NCSR. This is recognised by the NCSR Act 2016. |
Pseudonym |
This is a request to the NCSR by an eligible person, their personal representative or healthcare provider, who wants to use a pseudonym for the NCSR. A person is able to request that an alternative name be used by the NCSR when communicating with them, to protect privacy while continuing to participate in the NCSR. If the participant wishes to change their formal name, as recorded with Medicare, they must contact Medicare directly, as the NCSR obtains the person’s name information from Medicare. |
Request to the NCSR |
A request to the NCSR can be made in compliance with the NCSR Act 2016 to:
|
Resume Participation |
This is a request to the NCSR by an eligible person, their personal representative or healthcare provider who has opted out and who wishes to resume participation in a particular screening program facilitated by the NCSR. This is also referred to as withdrawing the opt out request. |
States and Territories Access number (STAN) and Register Identifier Number (RIN) |
The Register Identifier number is a unique number used to authenticate cervical screening providers who do not have access to a Medicare Provider number, when accessing the Portal or calling the NCSR Contact Centre to access participant information. Depending on the requirements of the state or territory, healthcare providers who deliver services across multiple location may have multiple Register Identifiers. |
